

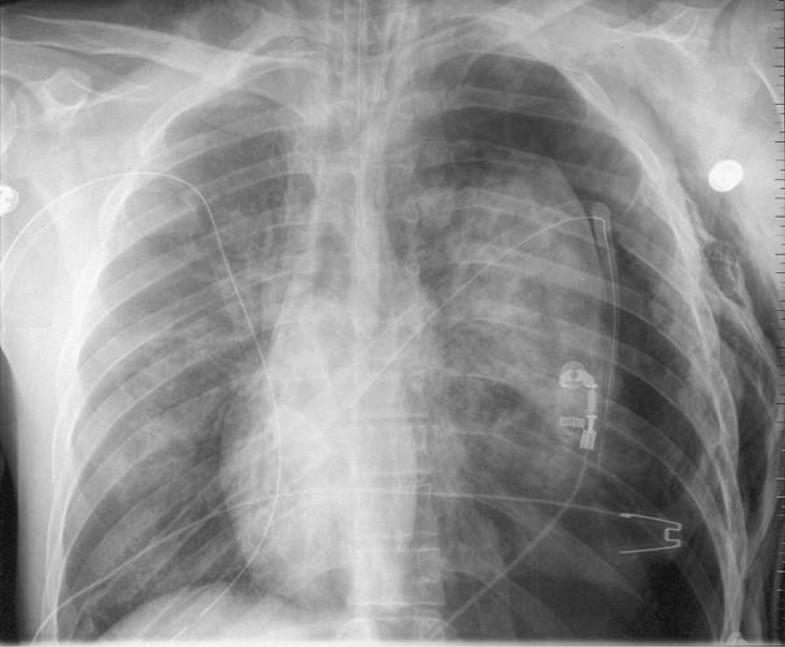
A dedicated trial using this device is now warranted. Our study has, however, shown early supportive evidence of safety in the attachment of an Atrium Pneumostat to an existing chest drain to ambulate patients with secondary spontaneous pneumothorax. “We do not advise use of pleural vent in patients with secondary spontaneous pneumothorax, and the encouraging results with pleural vent in primary spontaneous pneumothorax are not translatable to this population. This may be related to high rates of treatment failure with the pleural vent and a shorter length of stay in the standard arm than previously cited,” the researchers wrote. “In conclusion, there was no difference on length of stay between patients managed with ambulatory care compared to chest drain attached to underwater seal. Results also showed early and sustained improvements in breathlessness, chest pain and quality of life in both groups. Patients managed with the Atrium Pneumostat valve had no early treatment failures (median length of stay, 1.5 days). The rate of early treatment failure was higher among patients with a pleural vent compared with patients who received standard care (46% vs. Researchers observed no difference in length of stay during the first 30 days: median length of stay was 6 days in the ambulatory care group and 6 days in the standard care group ( P =. The primary outcome was hospital length of stay. In the ambulatory care group, 13 patients were managed with the Pleural Vent (Rocket Medical PLC) and eight were managed with the Atrium Pneumostat valve (Atrium Medical). Type of flutter valve used was based on whether a patient already had a chest tube in place at the time of randomization. We describe a case of benign truncal nonpalpable purpura in a man who had been treated with a new pulmonary mucus clearing device called a Flutter valve. Patients were randomly assigned to ambulatory care with flutter valve (n = 21 mean age, 63.4 years 67% men) or standard care with a chest tube attached to an underwater seal (n = 20 mean age, 68.1 years 85% men).
Pulmonary flutter valve free#
The open-label, randomized controlled trial included 41 patients with secondary spontaneous pneumothorax from March 2017 to March 2020. The flutter is a respiratory device that produces an oscillatory positive expiratory pressure that can be used as an alternative for removal of secretions. Free Express Shipping PEPE is a handheld mucus clearance device, like the Flutter device loosens mucus & clears the airways. So in pulmonary hypertension the atrial contraction may not be powerful enough to cause an ‘a’ notch in the pulmonary valve echocardiogram. “Pooled results from retrospective studies suggest that flutter valves may reduce hospital length of stay in this patient cohort.”ĭata were derived from Walker SP, et al. Walker, MD, clinical research fellow in the Academic Respiratory Unit at the University of Bristol School of Clinical Sciences at Southmead Hospital, Westbury-on-Trym, U.K., and colleagues wrote. “There are no published prospective studies describing use of flutter valves in patients with secondary spontaneous pneumothorax,” Steven P. Regarding airway clearance, one study used Flutter VRP1 40, one used Flutter valve TM 33, one used the UNIKO-TPEP ® 23, and two studies used oscillatory positive expiratory pressure (OPEP. There was no difference in hospital length of stay among patients with secondary spontaneous pneumothorax who received ambulatory care with a flutter valve or standard care, according to results published in the European Respiratory Journal. If you continue to have this issue please contact to Healio All rights reserved.We were unable to process your request. The Flutter Valve improves lung secretion removal, mucus production, respiratory mechanics, and arterial oxygenation in mechanically ventilated patients with respiratory infection, without causing clinically relevant hemodynamic repercussions.Ĭopyright © 2011 Elsevier Inc. Control intervention (CTRL) was normal ventilation in pressure controlled mode.Ĭompared with CTRL, FLUTTER improved sputum production (P. FLUTTER intervention consisted of connecting the Flutter Valve to the exhalation port of the mechanical ventilator. In a randomized crossover study, sputum production, respiratory mechanics, hemodynamics, and gas exchange were evaluated from 20 mechanically ventilated patients submitted to 2 interventions. This study was designed to evaluate whether an airway clearance protocol using the Flutter Valve can affect the therapeutic and physiologic outcomes in mechanically ventilated patients with pulmonary infection. The Flutter Valve (Varioraw SARL, Scandipharm Inc, Birmingham, AL) has proven efficacy in hypersecretive spontaneously ventilated patients.


 0 kommentar(er)
0 kommentar(er)
